Sale of talcum powder to be stopped globally by Johnson & Johnson, India yet to stop sale of asbestos laden talcum powder

August 12, 2022: ToxicsWatch Alliance (TWA) and Ban Asbestos Network of India (BANI) welcome the announcement of Johnson & Johnson, a multinational company headquartered in New Brunswick, New Jersey, USA to stop the sale of baby talc powder across the world including India from 2023. It is apparent that Indians will continue to be exposed to asbestos laden talcum powder throughout 2022 unless the Government of India acts to stop the sale of talcum powder with immediate effect. Companies like Johnson & Johnson have been insensitive towards public health for quite a long time. They have been practicing practising racism too. Johnson & Johnson and other companies which sell talcum powder should be made to stop the sale of adult talc powder besides baby talcum powder. TWA has been pursuing the demand for ban on sale of asbestos laden talcum powder with the National Human Rights Commission and Drugs Controller General of India (DCGI), Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Union Ministry of Health and Family Welfare for several years without success.
TWA and BANI demand that Government of India should promote use of cornstarch and stop the sale of talcum powder of all the companies now that Johnson & Johnson has decided to stop its sale across the globe including India. Other companies which are selling talcum powder to unsuspecting consumers in India too should be ordered to stop their sale to safeguard the public health.
When TWA approached Dr. V.G. Somani, Drugs Controller General of India (DCGI), Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Union Ministry of Health and Family Welfare in this regard, he informed that “as far as the asbestos in talcum powder is concerned, asbestos is already prohibited to be used in the cosmetic products as per the Indian Standards IS 4707 Part 2:2017. Further, recently, the BIS has amended Indian Standard, IS 1462 ‘Talc for Cosmetic Industry- Specification’, with regard to the requirement and test method for the absence of asbestos.” But the continued sale of asbestos laden talcum powder in India whose sale has been discontinued in North America shows that this law has not been enforced. DCGI is yet to address the complaint regarding ongoing exposure of Indians to hazardous asbestos mineral fibers contaminated talcum powder of Johnson & Johnson and other brands and enforce prohibition of the sale of talcum powder products to safeguard the health of residents and citizens of India.
In April 2022 when Johnson & Johnson’s shareholders voted against a proposal to stop sales of the talc baby powder in India and other non-North American markets, TWA and BANI had accused shareholders of Johnson & Johnson of practicing double standard and racism. In a classic case of double standard and racism, US shareholders of Johnson & Johnson had agreed to stop sale of asbestos laden talc powder in North America but had acquiesced to continue sale of toxic talc to countries like India. It is inhuman and immoral to knowingly expose humans to killer asbestos fibers. The claim of Johnson & Johnson that its “Baby Powder is safe, does not contain asbestos, and does not cause cancer” is an exercise to save itself from liabilities emerging out of fatal diseases caused by the consumers of its asbestos laden talc powder. In this regard it is relevant to recall that responding to questions about safety of talcum powder and whether talc contains harmful contaminants, such as asbestos, in January 2022, USA’s Food and Drugs Administration (USFDA) released a White Paper and technical appendices on testing methods for asbestos in cosmetic products containing talc. Talc is an ingredient used in many cosmetics, from baby powder to blush.
On May 19, 2020 Johnson & Johnson had announced that it will discontinue sale of its Talcum Powder products in North America. This announcement was aimed at safeguarding the health of residents and citizens of North America but not the residents and citizens of India and non-North American regions. It also announced that “the Company will wind down the commercialization of talc-based Johnson’s Baby Powder in the U.S. and Canada in the coming months. Existing inventory will continue to be sold through retailers until it runs out.” Now that it has agreed to stop sale of talc based powder across the globe, it emerges that it is and has been knowingly exposing Indians and non-North Americans to carcinogenic asbestos for years.
A study titled “Asbestos in commercial Indian talc” published in the American Journal of Industrial Medicine states that “this product study of various talcum powders marketed to combat prickly heat, purchased from Indian retailers both over‐the‐counter and online, demonstrates the ease of general population access to such products and the potential for significant exposure to asbestos. The analytical results of this study confirm that asbestos exposure of the Indian and potentially greater Southeast Asian populations is not limited to traditional occupational settings.” The findings of this study “imply that the asbestos‐related medical and public health implications to consider will need to extend to persons of both genders and all ages among this population group. This study’s confirmation of an underappreciated source of asbestos exposure, through personal care products, also highlights the risk that anyone within breathing range of these aerosolizeable, contaminated, talcum products incurs.” The authors of the study observe, “Until asbestos is also viewed as a hazard in India and banned, there will still be considerable risk to health.”
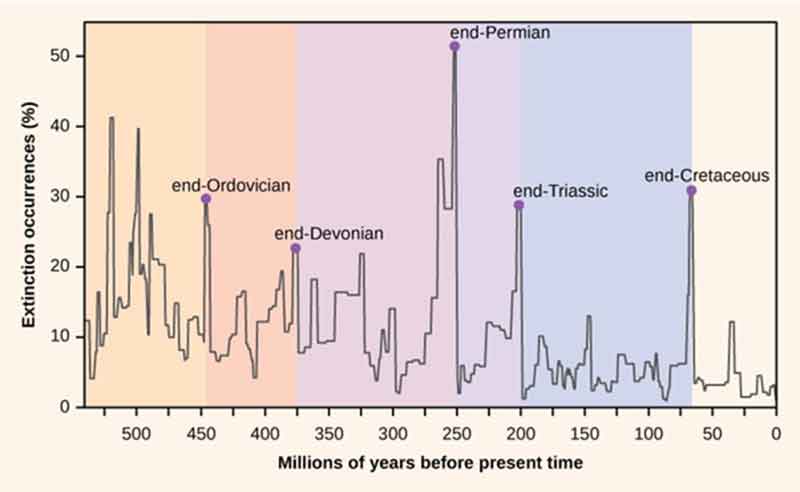
Notably, Word Health Organisation (WHO)’s International Agency for Cancer Research (IARC) has recognized the presence of asbestos in talcum powder. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans on Carbon Black, Titanium Dioxide, and Talc (2010) refers to the presence of asbestos in talcum powder. It also refers to “Use of talc for feminine hygiene”. The use of body powder for feminine hygiene can be estimated from the prevalence reported for controls in case–control studies that investigated the association between the use of cosmetic talc for feminine hygiene and the risk for ovarian cancer. It refers to exposure to respirable dust during the use of talcum powders on the face, body and babies. Talc is used as a surface lubricant on the majority of condoms manufactured; contact with condoms may also represent a direct means of exposure of the female genital tract to talc. Exposure to talc can also occur during surgical procedures when using powdered gloves. Talc particles were observed in the navels of small children, in the testes, on the vocal cords, in the urinary bladder tract and after removal of varicous veins. Besides this the Food Chemical Codex (2003) provides specifications for food-grade talc, including the statement that “talc derived from deposits that are known to contain associated asbestos is not food grade.” Under the voluntary guidelines initiated in 1976, the Cosmetic, Toiletry, and Fragrances Association stated that all cosmetic talc should contain at least 90% platy talc (hydrated magnesium silicate) that is free from detectable amounts of fibrous, asbestos minerals. Meanwhile, some 67 countries have banned all kinds of asbestos. World Health Organisation (WHO)’s recommendations have established the infectious nature of Covid-19, the same WHO has underlined that “All types of asbestos cause lung cancer, mesothelioma, cancer of the larynx and ovary, and asbestosis (fibrosis of the lungs).”[Reference: https://www.who.int/ipcs/assessment/public_health/asbestos/en/ and https://www.who.int/news-room/fact-sheets/detail/asbestos-elimination-of-asbestos-related-diseases.]
Fitzgerald et al observe, “With products of this nature being readily available and appealing to both genders, it is necessary to consider what the potential health risks and burdens of disease are for millions of exposed women of childbearing age and the children for whom they provide care. IARC has confirmed the causal association of asbestos with ovarian cancer and other cancers”.
TWA and BANI demand that CDSCO must undertake the enviro-occupational health audit of the workers who handle asbestos laden talcum powder in the manufacturing facilities of talcum powder products in general besides the health audit of the communities who are in the vicinity of such factories and recommend adequate compensation for those who are exposed to the carcinogenic mineral fibers and are suffering from asbestos related diseases. This will be also relevant for assessing the harm which the unsuspecting consumers continue to face. These consumers include all judges, legislators, officials, their children and grandchildren and the residents of India.
It is noteworthy that in an investigative report titled “Johnson & Johnson knew for decades that asbestos lurked in its Baby Powder” published on December 14, 2018 which too is relevant for protecting the human rights of Indians. The investigation was conducted by Reuters, a news agency. This investigative report is consistent with the findings of a study by India’s Industrial Toxicology Research Centre (IITR), Lucknow, a constituent laboratory of Council of Scientific & Industrial Research (CSIR), Ministry of Science and Technology, Government of India on “Exposure risk to contaminants in pharmaceutical and cosmetic powders” has found that “There are different types of cosmetic powders such as body powder, baby powder, face powder, eye shadow and powdered blush as well as pharmaceutical powders available in the market. Both the sexes of all age groups are using these powders. These are talc – based. Talc is a mineral product and often contaminated with asbestos fibres.”
The aim of the IITR study “was to investigate the safety of such powders being sold in the market, initially by analyzing the asbestos content. Five branded samples of talcum powder were analysed and all were found contaminated with asbestos fibres. Asbestos fibre contamination in these powders ranged from 10.3 – 15.4%. Fibre length study on two samples revealed that asbestos fibres were 22.8 – 34.7%, 48.2 – 55.1% and 17.1 – 22.1% in the range of <10 10=”” 20=”” and=”” m=””> 20µm, respectively. The study indicates risk of human exposure to asbestos through the use of naturally contaminated talcum powder. It is noteworthy that asbestos takes many years to cause asbestosis and carcinogenic malignancies which are irreversible. It also necessitates a regular monitoring and surveillance on all the cosmetic and pharmaceutical powders being marketed for asbestos contamination.” This has been published in the Annual Report 2005-2006 of IITR. IITR is accredited by National Accreditation Board for Testing and Calibration Laboratories (NABL) for chemical and biological testing and is recognized for GLP (Good Laboratory Practice) toxicity testing.[Reference:http://www.itrcindia.org/ITRC_Annual_Report_2005-06.pdf ]
The investigation by Reuters had corroborated the findings of IITR. This recent investigation was undertaken in the wake of three verdicts in New Jersey, California and St. Louis awarding compensation to plaintiffs who blamed asbestos-tainted Johnson & Johnson talc products for their mesothelioma, a type of cancer that develops from the thin layer of tissue that covers many of the internal organs. The connection between asbestos exposure and mesothelioma was discovered in the 1970s. The third verdict was a watershed in in St. Louis: The 22 plaintiffs were the first to succeed with a claim that asbestos-tainted Baby Powder and Shower to Shower talc, a longtime brand the company sold in 2012 that caused ovarian cancer, which is much more common than mesothelioma. The jury awarded them $4.69 billion in damages. Most of the talc cases have been brought by women with ovarian cancer who say they regularly used Johnson and Johnson talc products as a perineal antiperspirant and deodorant. The inclusion of ovarian cancer besides mesothelioma has broadened the potential liability of Johnson & Johnson, a 132 year old multinational medical devices, pharmaceutical and consumer packaged goods manufacturing company headquartered in New Brunswick, New Jersey, USA.
This announcement of Johnson & Johnson dated August 11, 2022 is of deep relevance for the public health of present and future generation of Indians given the fact that Johnson & Johnson company has admittedly been in India for the last 70 years. The company has brought many products in consumer healthcare, medical devices and pharmaceuticals. In 1947, Johnson & Johnson expanded into India, marketing Johnson’s Baby Powder. In September 1957, Johnson & Johnson incorporated as a legal entity in India. The production in its first manufacturing facility began in 1959 at the Johnson & Johnson India plant in Mulund, Mumbai, for Johnson’s Baby Powder and other specialized products. In 1968, the company introduced the Stayfree brand to India. A situation emerged wherein Johnson & Johnson reached almost every household in India.
In India, the import, manufacture, distribution and sale of cosmetics is regulated under the provisions of Drugs and Cosmetics Act, 1940 and Rules made thereunder. Schedule ‘S’ of the Drugs and Cosmetics Rules, 1945 specifies that the cosmetics in finished form shall conform to the India Standards specifications laid down from time to time by the Bureau of Indian Standards (BIS). The non-enforcement of these standards has created a situation where in the face of global outrage against asbestos laden talcum powder, these products continue to be in the Indian market unmindful of its disastrous public health consequences.
For Details: Dr. Gopal Krishna, ToxicsWatch Alliance (TWA)/Ban Asbestos Network of India (BANI), E-mail:[email protected] Web: www.asbestosfreeindia.org, www.toxicswatch.org